Electromagnetic Wave: Energy and Wavelength Relationship
The relationship between the wavelength (\( \lambda \)) and energy (\( E \)) of electromagnetic waves can be described using the following key formulas:
1. Energy and Frequency Relationship
The energy of an electromagnetic wave is directly proportional to its frequency (\( f \)). This relationship is given by Planck's equation:
\[
E = h f
\]
Where:
- \( E \) is the energy of the wave
- \( h \) is Planck's constant (\( 6.626 \times 10^{-34} \, \text{J} \cdot \text{s} \))
- \( f \) is the frequency of the wave
2. Frequency and Wavelength Relationship
The frequency of an electromagnetic wave is inversely related to its wavelength (\( \lambda \)), and this relationship is expressed as:
\[
c = f \lambda
\]
Where:
- \( c \) is the speed of light in a vacuum (\( 3.00 \times 10^8 \, \text{m/s} \))
- \( f \) is the frequency
- \( \lambda \) is the wavelength
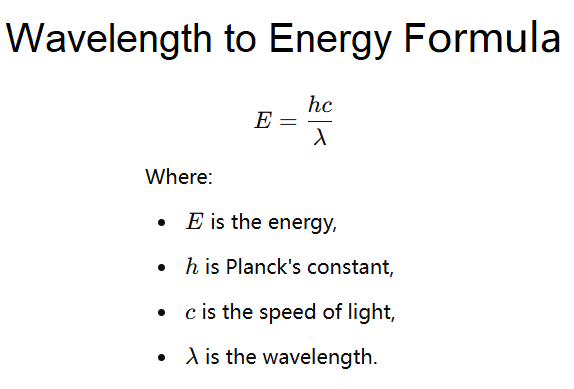
3. Combining the Two Relationships
By combining the above formulas, we can express the energy of an electromagnetic wave in terms of its wavelength:
\[
E = \frac{h c}{\lambda}
\]
Where:
- \( E \) is the energy
- \( h \) is Planck's constant
- \( c \) is the speed of light
- \( \lambda \) is the wavelength
Applications of Wavelength, Energy, and Frequency
These calculations are fundamental in many areas of physics:
- Electromagnetic Spectrum: Understanding the energy and frequency of different types of electromagnetic radiation (radio waves, microwaves, infrared, visible light, UV, X-rays, gamma rays).
- Quantum Mechanics: The wave-particle duality concept is crucial in quantum mechanics, and these formulas help relate the energy and frequency of photons to their wavelength.
- Telecommunications: In communication systems, the energy and frequency of waves play a critical role in signal transmission and detection.
 Home
Home
 Back
Back